Knowing that H blood vessels are lost via aging is a breakthrough as it may indicate that restoring type H blood vessels may be key to restoring the growth plate.
Lateral Synovial Joint Loading has been linked to type H blood vessel formation. Type H vessels are key for transport of cells. If we can reverse the process of Type H vessels to type L vessels perhaps that would reinitiate a natural process of growing taller.
Mechanical forces couple bone matrix mineralization with inhibition of angiogenesis to limit adolescent bone growth
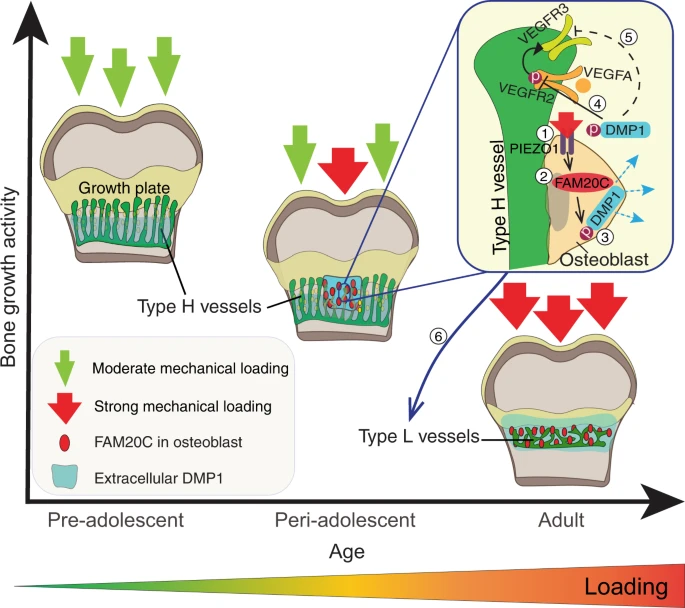
“Bone growth requires a specialised, highly angiogenic blood vessel subtype, so-called type H vessels, which pave the way for osteoblasts surrounding these vessels. At the end of adolescence, type H vessels differentiate into quiescent type L endothelium lacking the capacity to promote bone growth.{How do we reverse this process?} Until now, the signals that switch off type H vessel identity and thus limit adolescent bone growth have remained ill defined. Here we show that mechanical forces, associated with increased body weight at the end of adolescence, trigger the mechanoreceptor PIEZO1 and thereby mediate enhanced production of the kinase FAM20C in osteoblasts.{so if we are underweight type H blood vessels will stay around longer?} FAM20C, the major kinase of the secreted phosphoproteome, phosphorylates dentin matrix protein 1, previously identified as a key factor in bone mineralization. Thereupon, dentin matrix protein 1 is secreted from osteoblasts in a burst-like manner. Extracellular dentin matrix protein 1 inhibits vascular endothelial growth factor signalling by preventing phosphorylation of vascular endothelial growth factor receptor 2. Hence, secreted dentin matrix protein 1 transforms type H vessels into type L to limit bone growth activity and enhance bone mineralization.{so a dentin matrix protein 1 inhibitor may be a possible mechanism in which to grow taller?} The discovered mechanism may suggest new options for the treatment of diseases characterised by aberrant activity of bone and vessels such as osteoarthritis, osteoporosis and osteosarcoma.”
” Type H vessels are located exclusively at the sites of active bone growth, namely the ossification front (OF) and periosteum, and they are organised in columnar structure. Notch signalling, Hypoxia-inducible factor 1-alpha (HIF1α) activity, blood flow speed and slit guidance ligand 3 (SLIT3) were shown to support type H vessel formation”
“endothelial cells in bone require integrins for their proper functioning and maintenance, which highlights extracellular matrix (ECM) proteins as important factors for endothelium activity”
” we performed laser microdissection (LMD) of single capillary with associated surrounding cells in the OF of juvenile (4-week-old) and adult (12-week-old) mice.”
“Our detailed analysis of DMP1 protein localisation throughout postnatal bone development showed that until 5 weeks of age, the protein was mainly localised at the base of the zone of type H vessels in the [Ossification front]”
“DMP1 accumulation in the OF[Ossification Front] centre at 5.5–6 weeks correlated with a decrease in endomucin (EMCN) intensity and reduced amounts of MMP9 at the OF-GP border. This was associated with the disappearance of bulges and columnar structure of the vessels”
They found though that the DMP1 knockout femurs were shorter at a certain age point but perhaps a reduction in DMP1 would lead to slower more sustained growth.
“DMP1 secretion correlates positively with body weight and coincides spatiotemporally with FAM20C upregulation”
“mechanical loading through increasing body weight or/and muscle contractions, directly or indirectly, controls DMP1 secretion along with FAM20C upregulation.”
“, body weight-associated mechanical loading triggers the mechanoreceptor PIEZO1 to enhance the production of FAM20C kinase in osteoblasts, which induces a burst in DMP1 secretion into ECM. Second, large amounts of extracellular DMP1 inhibit VEGF signalling in the OF and transform highly angiogenic type H vessels into quiescent type L vasculature to limit bone growth activity. In parallel, extracellular DMP1 leads to rapid matrix mineralisation and strengthening of long bones”
Source link
